|
 |
 |
|
||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
 | ||||||||||||||||||||||||
Confocal Microscopy Image Gallery
Embryonic Albino Swiss Mouse Fibroblast Cells (3T3 Line)
Established by George Todaro and Howard Green in 1962 from disaggregated Swiss mouse (Mus musculus) embryo tissue, the 3T3 cell line is a standard fibroblast cell line used in a wide spectrum of research and industrial biomedical applications. Variants of the initial cell line have been tested and found negative for ectromelia virus (mousepox), but most are susceptible to polyoma and simian virus 40 (SV40).

In addition, 3T3 cells are negative for reverse transcriptase, indicating the lack of integral retrovirus genomes. Within the cytoplasm, lysophosphatidylcholine (lyso-PC) induces AP-1 activity and c-jun N-terminal kinase activity (JNK1) by a protein kinase C-independent pathway. Contact inhibited, a confluent monolayer of 3T3 cells yields approximately 40,000 cells per square centimeter.
At the time of their establishment, 3T3 cells were different than most other cell lines in regard to the fact they did not induce tumors to develop when injected into murine species. However, 3T3 cells, it was quickly realized, were not normal cells either, since they are capable of growing indefinitely. In fact, the unusual behavior of the line enabled researchers to make a clear distinction for the first time between immortal cells and cells that have the ability to form tumors; previous to studies of the 3T3 line, it was believed that these characteristics were necessarily synonymous with one another. However, due to examination of 3T3 cells and subsequent research it has become widely accepted that for immortalization of cells to take place, telomere shortening, which can instigate chromosomal rearrangements, must be overcome, a process that is not necessarily related to a cell's ability to undergo oncogenic transformation.
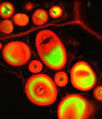
The isolated 3T3 cell presented in the digital image above was resident in an adherent culture stained for F-actin with Alexa Fluor 546 conjugated to phalloidin, and for DNA with the red-absorbing dye TO-PRO-3. In addition, the culture was immunofluorescently labeled with Alexa Fluor 488 conjugated to antibodies that target alpha-tubulin, a major component of the microtubule network. Images were recorded with a 60x oil immersion objective using a zoom factor of 2.5 and sequential scanning with the 488-nanometer spectral line of an argon-ion laser, the 543-nanometer line from a green helium-neon laser, and the 633-nanometer line of a red helium-neon laser. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
Additional Confocal Images of Embryonic Swiss Mouse Fibroblast (3T3) Cells
Albino Swiss Mouse Embryo Fibroblast Cells with Alexa Fluor 488 and the DNA Probe TO-PRO-3 - A log phase culture of 3T3 cells was immunofluorescently labeled with primary anti-tubulin mouse monoclonal antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 488. The cells were simultaneously stained for DNA with TO-PRO-3 and imaged using argon-ion and helium-neon lasers.
Tubulin, Actin, and DNA Distribution in 3T3 Cells - A culture of 3T3 cells was immunofluorescently labeled with primary anti-tubulin mouse monoclonal antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 488. In addition, the cells were simultaneously probed for DNA with the red-absorbing probe TO-PRO-3, and for the cytoskeletal filamentous actin network with Alexa Fluor 568 conjugated to phalloidin.
Swiss Mouse Embryo Fibroblast Cells with MitoTracker Deep Red 633, Texas Red, and SYTOX Green - A log phase culture of embryonic Swiss mouse fibroblast cells was stained with MitoTracker Deep Red 633, Texas Red conjugated to phalloidin, and SYTOX Green, which target the intracellular mitochondrial network, cytoskeletal actin filaments, and nuclei, respectively. High signal levels from all three of the fluorophores employed to stain the culture are present.
Using Concanavalin A to Probe the Endoplasmic Reticulum Network in Albino Swiss Mouse Embryo Cells - A single Swiss mouse embryo fibroblast cell resident in an adherent culture and stained with Alexa Fluor 488 conjugated to the lectin concanavalin A is presented in this section. Concanavalin A selectively binds to alpha-mannopyranosyl and alpha-glucopyranosyl residues in glycoproteins found in the endoplasmic reticulum. The specimen was also counterstained with TO-PRO-3, targeting DNA in the nuclei.
Imaging the Mitochondrial Network in Monolayer 3T3 Fibroblast Cell Cultures - A monolayer culture of Swiss mouse embryo cells was treated with MitoTracker Red CMXRos in medium containing 15 percent Cosmic calf serum, fixed with the same medium containing 3.7 percent paraformaldehyde, permeabilized with 0.2 percent Triton X-100, and then counterstained with SYTOX Green, targeting DNA in the nuclei.
Targeting the Nuclear Histones and Cytoplasmic Peroxisomes in Albino Swiss Mouse Fibroblast Cells - In a double immunofluorescence labeling experiment, an adherent culture of Swiss mouse embryo cells was treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively, to target the nuclear histone proteins and peroxisomes.
Imaging the Cytoskeletal Network in 3T3 Cell Cultures - The isolated 3T3 cell presented in this section was resident in an adherent culture stained for F-actin with Alexa Fluor 546 conjugated to phalloidin, and for DNA with the red-absorbing dye TO-PRO-3. In addition, the culture was immunofluorescently labeled with Alexa Fluor 488 conjugated to antibodies that target alpha-tubulin, a major component of the microtubule network.
3T3 Fibroblast Cells with Alexa Fluor 488 and TO-PRO-3 - Similar to one of the gallery entries listed above, an adherent log phase culture of 3T3 cells was immunofluorescently labeled with primary anti-tubulin mouse monoclonal antibodies followed by goat anti-mouse secondary antibodies conjugated to Alexa Fluor 488. The cells were simultaneously stained for DNA with TO-PRO-3 and imaged using argon-ion and helium-neon lasers.
Microtubule Network in Albino Swiss Mouse Fibroblast Cells - In order to visualize the microtubules present in a culture of 3T3 fibroblasts, the cells were immunofluorescently labeled with anti-tubulin mouse monoclonal primary antibodies followed by goat anti-mouse Fab fragments conjugated to Alexa Fluor 555. In addition, the cells were labeled for DNA in the nucleus with the red-absorbing dye TO-PRO-3.
Distribution of Histone Proteins and Peroxisomes in 3T3 Cultures - Nuclear histone proteins were targeted in the culture of 3T3 cells presented in this section with mouse anti-histone (pan) monoclonal antibodies, which were imaged with goat anti-mouse Fab fragments conjugated to Alexa Fluor 568 (labeling the nucleus). The specimen was simultaneously labeled for peroxisomes with Alexa Fluor 488 conjugated to goat secondary antibodies that target rabbit anti-PMP 70 (peroxisomal membrane protein 70).
Targeting Filamentous Actin in Mouse Fibroblasts with a Phallotoxin - A log phase culture of 3T3 cells was treated with a cocktail of mouse anti-histones (pan) and rabbit anti-PMP 70 (peroxisomal membrane protein) primary antibodies, followed by goat anti-mouse and anti-rabbit secondary antibodies conjugated to Alexa Fluor 568 and Alexa Fluor 488, respectively, to target the nuclear histone proteins and peroxisomes. In addition, the F-actin network was labeled with Alexa Fluor 633 (pseudocolored blue) conjugated to phalloidin, a phallotoxin isolated from the toxic death cap mushroom.
Contributing Authors
Nathan S. Claxton, Shannon H. Neaves, and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.